오므론에 자주 문의 주신 질문사항은 FAQ로 정리하여
빠른 답변을 제공하고 있습니다. FAQ에서 빠르게 답변을 확인해보세요.
Clinical
Validation
국제공인기관의 프로토콜을 통과한 오므론 혈압계는 최고 수준의 정밀도로 신뢰성을 충족시키며,
의료현장에서 사용하는 의료기기와 동일하게 건강 모니터링이 가능합니다.
많은 분들이 혈압계를 선택하실 때 가장 중요하게 생각하는 것이 ‘정확도’입니다.
혈압계의 정확도는 국제적인 검증 프로토콜을 준수하였는지의 여부로 확인할 수 있습니다.
프로토콜 검증은 독립적인 기관인 AAMI (Association for the Advancement of Medical Instrumentation) 및
유럽 고혈압 협회 (European Society for Hypertension, ESH) 와 같은 공식적인 기관에서 엄격한 테스트로 평가한 후, 프로토콜 통과 여부가 결정됩니다.
국내에서 판매되는 오므론의 모든 혈압계는 적어도 1개 이상의 국제적 검증 프로토콜을 준수하여 압력 정확도를 검증받았습니다.
-
1. dabl 목록에서 Ctrl+F 키를 누른 후, 입력창에 원하는 모델명을 입력하여 검색해주세요.
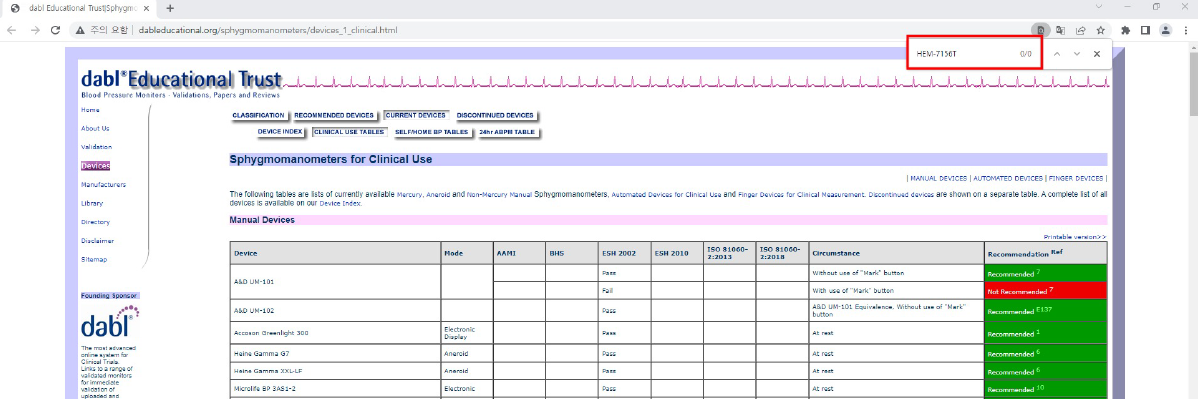
-
2. dabl에서 찾는 모델이 없는 경우, 하단 목록에서 Equivalence Certificate (동등성 증명서) 또는
Clinical Study (임상연구)에 쓰인 적용모델을 찾아주세요.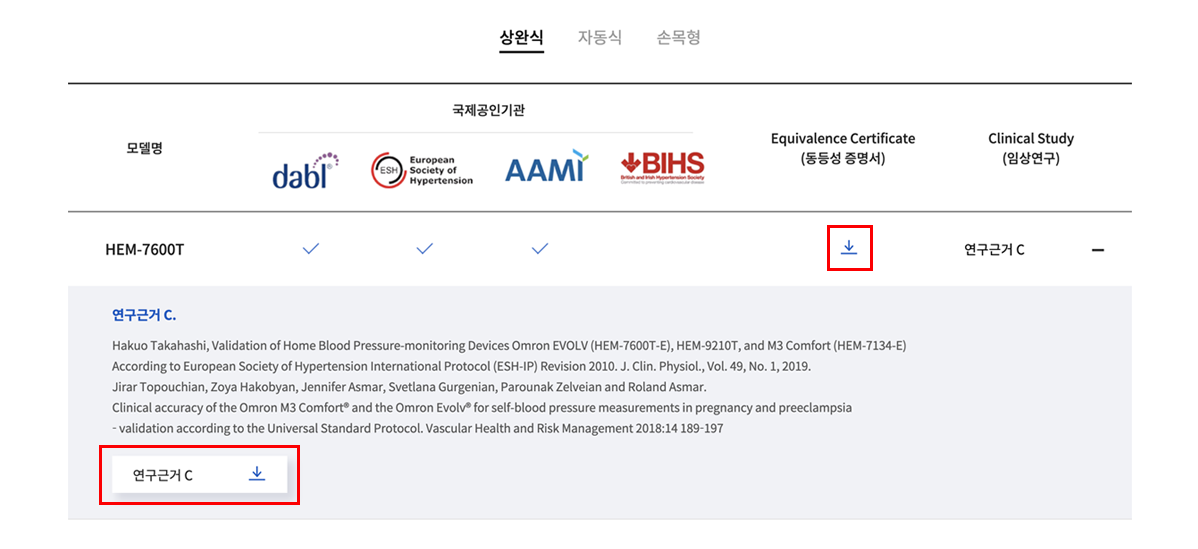
-
3. 다시 dabl에서 Ctrl+F 키를 누른 후 검색창에서 적용모델을 입력하여 검색해주세요.
예) HEM-7156T의 동등성 모델은 HEM-7134-E 입니다. HEM-7134-E는 dabl 리스트에서 확인할 수 있습니다.
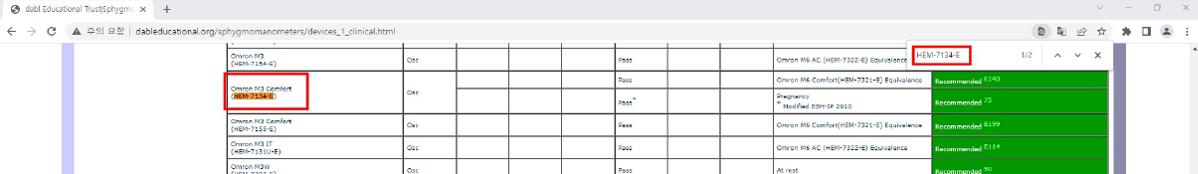
* 동일한 모델이라고 할지라도 국가별 모델명이 상이할 수 있습니다.
- 모델명
-
국제공인기관




- Equivalence Certificate
(동등성 증명서) - Clinical Study
(임상연구)
-
연구근거 C.
Hakuo Takahashi, Validation of Home Blood Pressure-monitoring Devices Omron EVOLV (HEM-7600T-E), HEM-9210T, and M3 Comfort (HEM-7134-E)
According to European Society of Hypertension International Protocol (ESH-IP) Revision 2010. J. Clin. Physiol., Vol. 49, No. 1, 2019.
Jirar Topouchian, Zoya Hakobyan, Jennifer Asmar, Svetlana Gurgenian, Parounak Zelveian and Roland Asmar.
Clinical accuracy of the Omron M3 Comfort® and the Omron Evolv® for self-blood pressure measurements in pregnancy and preeclampsia
– validation according to the Universal Standard Protocol. Vascular Health and Risk Management 2018:14 189–197 -
연구근거 J.
Hakuo Takahashi, Kanako Saito, Nobuki Yakura. Validation of Omron HEM-9200T, a home blood pressure monitoring device for the upper arm, according to the American National Standards Institute/Association for the Advancement of Medical Instrumentation/International Organization for Standardization 81060-2:2013 protocol
연구근거 K.Hakuo Takahashi, Toyohiko Yokoi and Masamichi Yoshika , Validation of the OMRON HEM-7130 upper arm blood pressure monitor, in oscillometry mode, for clinic use and self measurement in a general population, according to the European Society of Hypertension International Protocol revision 2010
-
연구근거 H.
Validation of two automatic devices for the self measurement of blood pressure according to the ANSI/AAMI/ISO81060-2:2009 guidelines:
the Omron BP765 (HEM-7311-ZSA) and the Omron BP760N (HEM-7320-Z)연구근거 I.Validation of the Omron HEM-7320-LA, upper arm blood pressure monitor with Intelli Wrap Technology Cuff HEM-FL1 for self-measurement and clinic
use according to the European Society of Hypertension International Protocol revision 2010 in the Mexican population -
연구근거 H.
Validation of two automatic devices for the self measurement of blood pressure according to the ANSI/AAMI/ISO81060-2:2009 guidelines:
the Omron BP765 (HEM-7311-ZSA) and the Omron BP760N (HEM-7320-Z)연구근거 I.Validation of the Omron HEM-7320-LA, upper arm blood pressure monitor with Intelli Wrap Technology Cuff HEM-FL1 for self-measurement and clinic
use according to the European Society of Hypertension International Protocol revision 2010 in the Mexican population -
연구근거 H.
Validation of two automatic devices for the self measurement of blood pressure according to the ANSI/AAMI/ISO81060-2:2009 guidelines:
the Omron BP765 (HEM-7311-ZSA) and the Omron BP760N (HEM-7320-Z)연구근거 I.Validation of the Omron HEM-7320-LA, upper arm blood pressure monitor with Intelli Wrap Technology Cuff HEM-FL1 for self-measurement and clinic
use according to the European Society of Hypertension International Protocol revision 2010 in the Mexican population -
연구근거 H.
Validation of two automatic devices for the self measurement of blood pressure according to the ANSI/AAMI/ISO81060-2:2009 guidelines:
the Omron BP765 (HEM-7311-ZSA) and the Omron BP760N (HEM-7320-Z)연구근거 I.Validation of the Omron HEM-7320-LA, upper arm blood pressure monitor with Intelli Wrap Technology Cuff HEM-FL1 for self-measurement and clinic
use according to the European Society of Hypertension International Protocol revision 2010 in the Mexican population -
연구근거 H.
Validation of two automatic devices for the self measurement of blood pressure according to the ANSI/AAMI/ISO81060-2:2009 guidelines:
the Omron BP765 (HEM-7311-ZSA) and the Omron BP760N (HEM-7320-Z)연구근거 I.Validation of the Omron HEM-7320-LA, upper arm blood pressure monitor with Intelli Wrap Technology Cuff HEM-FL1 for self-measurement and clinic
use according to the European Society of Hypertension International Protocol revision 2010 in the Mexican population -
연구근거 H.
Validation of two automatic devices for the self measurement of blood pressure according to the ANSI/AAMI/ISO81060-2:2009 guidelines:
the Omron BP765 (HEM-7311-ZSA) and the Omron BP760N (HEM-7320-Z)연구근거 I.Validation of the Omron HEM-7320-LA, upper arm blood pressure monitor with Intelli Wrap Technology Cuff HEM-FL1 for self-measurement and clinic
use according to the European Society of Hypertension International Protocol revision 2010 in the Mexican population -
연구근거 J.
Hakuo Takahashi, Kanako Saito, Nobuki Yakura. Validation of Omron HEM-9200T, a home blood pressure monitoring device for the upper arm, according to the American National Standards Institute/Association for the Advancement of Medical Instrumentation/International Organization for Standardization 81060-2:2013 protocol
-
연구근거 A.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of three automatic devices for the self-measurement of blood pressure ccording to the European
Society of Hypertension International Protocol revision 2010: the Omron HEM-7130, HEM-7320F, and HEM-7500F. Blood Pressure Monitoring 2015, 20:92–97연구근거 J.Hakuo Takahashi, Kanako Saito, Nobuki Yakura. Validation of Omron HEM-9200T, a home blood pressure monitoring device for the upper arm, according to the American National Standards Institute/Association for the Advancement of Medical Instrumentation/International Organization for Standardization 81060-2:2013 protocol
-
연구근거 A.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of three automatic devices for the self-measurement of blood pressure ccording to the European
Society of Hypertension International Protocol revision 2010: the Omron HEM-7130, HEM-7320F, and HEM-7500F. Blood Pressure Monitoring 2015, 20:92–97연구근거 J.Hakuo Takahashi, Kanako Saito, Nobuki Yakura. Validation of Omron HEM-9200T, a home blood pressure monitoring device for the upper arm, according to the American National Standards Institute/Association for the Advancement of Medical Instrumentation/International Organization for Standardization 81060-2:2013 protocol
-
연구근거 A.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of three automatic devices for the self-measurement of blood pressure ccording to the European
Society of Hypertension International Protocol revision 2010: the Omron HEM-7130, HEM-7320F, and HEM-7500F. Blood Pressure Monitoring 2015, 20:92–97연구근거 J.Hakuo Takahashi, Kanako Saito, Nobuki Yakura. Validation of Omron HEM-9200T, a home blood pressure monitoring device for the upper arm, according to the American National Standards Institute/Association for the Advancement of Medical Instrumentation/International Organization for Standardization 81060-2:2013 protocol
-
연구근거 A.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of three automatic devices for the self-measurement of blood pressure ccording to the European
Society of Hypertension International Protocol revision 2010: the Omron HEM-7130, HEM-7320F, and HEM-7500F. Blood Pressure Monitoring 2015, 20:92–97연구근거 J.Hakuo Takahashi, Kanako Saito, Nobuki Yakura. Validation of Omron HEM-9200T, a home blood pressure monitoring device for the upper arm, according to the American National Standards Institute/Association for the Advancement of Medical Instrumentation/International Organization for Standardization 81060-2:2013 protocol
-
연구근거 A.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of three automatic devices for the self-measurement of blood pressure ccording to the European
Society of Hypertension International Protocol revision 2010: the Omron HEM-7130, HEM-7320F, and HEM-7500F. Blood Pressure Monitoring 2015, 20:92–97연구근거 J.Hakuo Takahashi, Kanako Saito, Nobuki Yakura. Validation of Omron HEM-9200T, a home blood pressure monitoring device for the upper arm, according to the American National Standards Institute/Association for the Advancement of Medical Instrumentation/International Organization for Standardization 81060-2:2013 protocol
-
연구근거 A.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of three automatic devices for the self-measurement of blood pressure ccording to the European
Society of Hypertension International Protocol revision 2010: the Omron HEM-7130, HEM-7320F, and HEM-7500F. Blood Pressure Monitoring 2015, 20:92–97 -
연구근거 A.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of three automatic devices for the self-measurement of blood pressure ccording to the European
Society of Hypertension International Protocol revision 2010: the Omron HEM-7130, HEM-7320F, and HEM-7500F. Blood Pressure Monitoring 2015, 20:92–97 -
연구근거 A.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of three automatic devices for the self-measurement of blood pressure ccording to the European
Society of Hypertension International Protocol revision 2010: the Omron HEM-7130, HEM-7320F, and HEM-7500F. Blood Pressure Monitoring 2015, 20:92–97 -
연구근거 A.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of three automatic devices for the self-measurement of blood pressure ccording to the European
Society of Hypertension International Protocol revision 2010: the Omron HEM-7130, HEM-7320F, and HEM-7500F. Blood Pressure Monitoring 2015, 20:92–97 -
연구근거 A.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of three automatic devices for the self-measurement of blood pressure ccording to the European
Society of Hypertension International Protocol revision 2010: the Omron HEM-7130, HEM-7320F, and HEM-7500F. Blood Pressure Monitoring 2015, 20:92–97 -
연구근거 A.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of three automatic devices for the self-measurement of blood pressure ccording to the European
Society of Hypertension International Protocol revision 2010: the Omron HEM-7130, HEM-7320F, and HEM-7500F. Blood Pressure Monitoring 2015, 20:92–97 -
연구근거 B.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of two automatic devices for the self-measurement of blood pressure
according to the ANSI /AA MI/IS O81060-2:2009 guidelines:the Omron BP765 (HEM-7311-ZSA ) and the Omron BP760N (HEM-7320-Z).
Vascular Health and Risk Management 2015:11 49–53 -
연구근거 B.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of two automatic devices for the self-measurement of blood pressure
according to the ANSI /AA MI/IS O81060-2:2009 guidelines:the Omron BP765 (HEM-7311-ZSA ) and the Omron BP760N (HEM-7320-Z).
Vascular Health and Risk Management 2015:11 49–53 -
연구근거 H.
Validation of two automatic devices for the self measurement of blood pressure according to the ANSI/AAMI/ISO81060-2:2009 guidelines:
the Omron BP765 (HEM-7311-ZSA) and the Omron BP760N (HEM-7320-Z) -
연구근거 H.
Validation of two automatic devices for the self measurement of blood pressure according to the ANSI/AAMI/ISO81060-2:2009 guidelines:
the Omron BP765 (HEM-7311-ZSA) and the Omron BP760N (HEM-7320-Z) -
연구근거 A.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of three automatic devices for the self-measurement of blood pressure ccording to the European
Society of Hypertension International Protocol revision 2010: the Omron HEM-7130, HEM-7320F, and HEM-7500F. Blood Pressure Monitoring 2015, 20:92–97 -
연구근거 A.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of three automatic devices for the self-measurement of blood pressure ccording to the European
Society of Hypertension International Protocol revision 2010: the Omron HEM-7130, HEM-7320F, and HEM-7500F. Blood Pressure Monitoring 2015, 20:92–97 -
연구근거 A.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of three automatic devices for the self-measurement of blood pressure ccording to the European
Society of Hypertension International Protocol revision 2010: the Omron HEM-7130, HEM-7320F, and HEM-7500F. Blood Pressure Monitoring 2015, 20:92–97 -
연구근거 A.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of three automatic devices for the self-measurement of blood pressure ccording to the European
Society of Hypertension International Protocol revision 2010: the Omron HEM-7130, HEM-7320F, and HEM-7500F. Blood Pressure Monitoring 2015, 20:92–97 -
연구근거 A.
Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of three automatic devices for the self-measurement of blood pressure ccording to the European
Society of Hypertension International Protocol revision 2010: the Omron HEM-7130, HEM-7320F, and HEM-7500F. Blood Pressure Monitoring 2015, 20:92–97
- 모델명
-
국제공인기관




- Equivalence Certificate
(동등성 증명서) - Clinical Study
(임상연구)
-
HBP-1100
연구근거 D.Linghui Meng, Di Zhao, Yan Pan, Wenqing Ding, Qing Wei, Hua Li, Pingjin Gao and Jie Mi. Validation of Omron HBP-1300 professional blood pressure monitor
based on auscultation in children and adults. BMC Cardiovascular Disorders (2016) 16:9 Lamaan Abbud, Diane Nzelu, Mariam Salaria, Polly Kay and Nikos A. Kametas.
Validation of the Omron HBP-1300 in pregnancy for medium-arm and large-arm circumferences according to the British Hypertension Society protocol.
Blood Pressure Monitoring 2018, 23:277–280 -
HBP-1300
연구근거 D.Linghui Meng, Di Zhao, Yan Pan, Wenqing Ding, Qing Wei, Hua Li, Pingjin Gao and Jie Mi. Validation of Omron HBP-1300 professional blood pressure monitor
based on auscultation in children and adults. BMC Cardiovascular Disorders (2016) 16:9 Lamaan Abbud, Diane Nzelu, Mariam Salaria, Polly Kay and Nikos A. Kametas.
Validation of the Omron HBP-1300 in pregnancy for medium-arm and large-arm circumferences according to the British Hypertension Society protocol.
Blood Pressure Monitoring 2018, 23:277–280 -
HBP-1320
연구근거 A.Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of three automatic devices for the self-measurement of blood pressure ccording to the European
Society of Hypertension International Protocol revision 2010: the Omron HEM-7130, HEM-7320F, and HEM-7500F. Blood Pressure Monitoring 2015, 20:92–97연구근거 I.Validation of the Omron HEM-7320-LA, upper arm blood pressure monitor with Intelli Wrap Technology Cuff HEM-FL1 for self-measurement and clinic
use according to the European Society of Hypertension International Protocol revision 2010 in the Mexican population -
HBP-9020
연구근거 F.Validation of the accuracy of the HBP-9020/HBP-9021 in adults and adolescents using the AAMI Protocol ANSI/AAMI/ISO 81060-2:2009(1).
Clinical Evaluation Report Of Professional Blood Pressure Monitor HBP-9020/HBP-9021. 11/2/2011 -
연구근거 H.
Validation of the Omron HBP-9031C blood pressure monitor for clinics and hospitals according to the ANSI/AAMI/
ISO 81060-2:2013 protocol Kanako Saito1, Yukiko Hishiki1 and Hakuo Takahashi2 -
HEM-1000
연구근거 E.Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of home blood pressure monitoring devices, Omron HEM-1020 and Omron i-Q132(HEM-1010-E),
according to the European Society of Hypertension International Protocol. Blood Pressure Monitoring 2011, 16:203–207 -
HEM-1020
연구근거 E.Hakuo Takahashi, Masamichi Yoshika and Toyohiko Yokoi. Validation of home blood pressure monitoring devices, Omron HEM-1020 and Omron i-Q132(HEM-1010-E),
according to the European Society of Hypertension International Protocol. Blood Pressure Monitoring 2011, 16:203–207
- 모델명
-
국제공인기관




- Equivalence Certificate
(동등성 증명서) - Clinical Study
(임상연구)
-
연구근거 G.
Validation of two automatic devices, Omron HEM-6232T and HEM-6181, for self-measurement of blood pressure at the wrist according to the ANSI/AAMI/ISO 81060-2:2013 protocol
and the European Society of Hypertension International Protocol revision 2010 -
HEM-6181
연구근거 G.Validation of two automatic devices, Omron HEM-6232T and HEM-6181, for self-measurement of blood pressure at the wrist according to the ANSI/AAMI/ISO 81060-2:2013 protocol
and the European Society of Hypertension International Protocol revision 2010 -
HEM-6232T
연구근거 G.Validation of two automatic devices, Omron HEM-6232T and HEM-6181, for self-measurement of blood pressure at the wrist according to the ANSI/AAMI/ISO 81060-2:2013 protocol
and the European Society of Hypertension International Protocol revision 2010 -
연구근거 G.
Validation of two automatic devices, Omron HEM-6232T and HEM-6181, for self-measurement of blood pressure at the wrist according to the ANSI/AAMI/ISO 81060-2:2013 protocol
and the European Society of Hypertension International Protocol revision 2010




